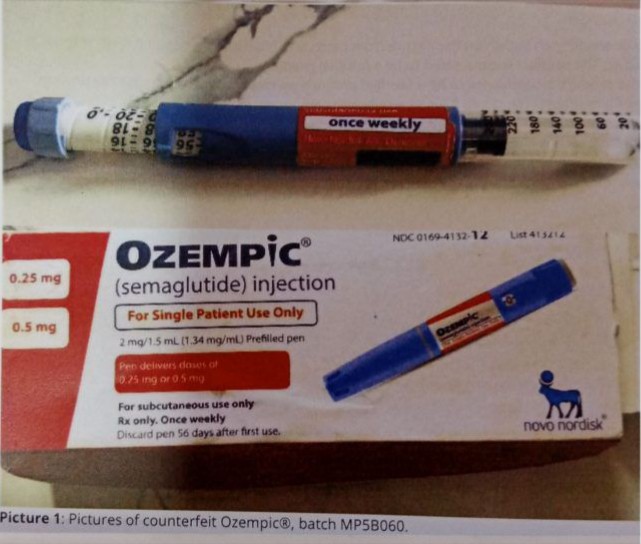
The National Agency for Food and Drugs Administration and Control (NAFDAC) has warned the public against one batch of falsified ozempic (Semaglutide) injection pen that has been identified in Nigeria.
Ozempic (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is an injectable prescription medicine.
It is a once-weekly medicine for adults with type 2 diabetes used to improve blood sugar, along with diet and exercise.
In a statement, NAFDAC said Novo Nordisk, the marketing authorisation holder (MAH) of the product, confirmed that the ozempic injection pen with batch number MP5B060 is falsified following an enquiry received from a customer about the product.
Advertisement
The agency said although no sample was returned to Novo Nordisk affiliate in Nigeria for investigation, the picture of the falsified product provided was scrutinised and the image of the pen in the picture was observed to be different from the one in the genuine product.
“According to the MAH, there were numerous cases related to the issue of falsified Ozempic originating from the Middle East and Russia. The affected countries include Azerbaijan, Egypt, Iraq, Jordan, Lebanon, Turkey, Uzbekistan and Russia,” NAFDAC said.
“Investigation conducted on the received falsified pens concludes that the pens are relabelled Apidra Solostar pen. The content of one of the pens was analysed and found to contain the fast-action insulin glulisin which is believed to be the case in all the falsified Ozempic pens identified. This implies the content of the falsified pens is entirely different from the genuine product.
Advertisement
“The main difference between the counterfeit product and the genuine product is that the genuine Novo Nordisk Ozempic pens do not extend or increase in length when setting the dose. The scale drum increments are fixed doses, such as 0.25 mg, 0.5 mg, and 1.0 mg for Ozempic pens.
“Although the product is not in NAFDAC database, it is likely that it might have been distributed in the country through informal markets. NAFDAC implores importers, distributors, retailers and healthcare providers and patients to always exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale and administration or use of falsified or substandard medicinal products. All medical products must be obtained from authorised/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
“If you have this falsified product, please DO NOT use it. If you, or someone you know, have used the product or suffered any adverse reaction/event after use, you are advised to seek immediate medical advice from a qualified healthcare professional.
“Healthcare professionals and consumers are advised to report any suspicion of adverse drug reaction, substandard and falsified medicines to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: [email protected]”
Advertisement
Add a comment