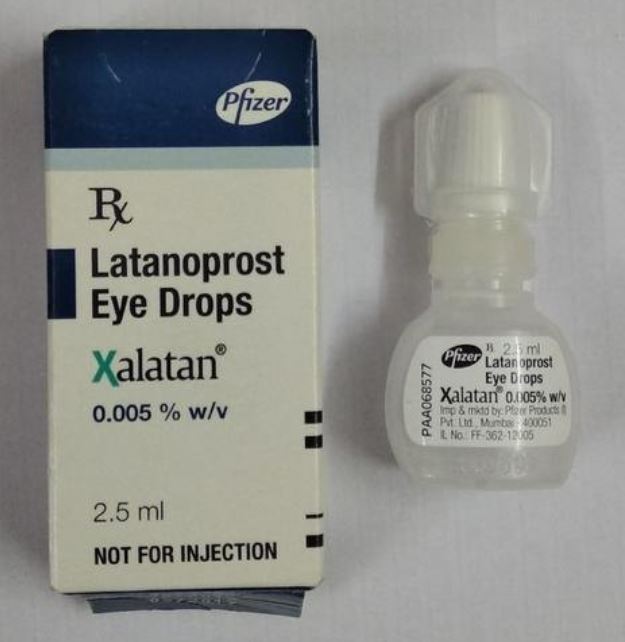
Pfizer, the American pharmaceutical company, is recalling batches of its Xalatan 0.005%w/v eye drops solution used in treating glaucoma.
In a notice sent to doctors and seen by TheCable, the company said the decision to recall was reached after some counterfeit products were found within the legitimate supply chain.
“Please be advised that Pfizer is recalling, at the patient level, the below lots of Xalatan 0.005%w/v Eye drops solution (the “Product Lots”) from distribution in Nigeria,” it said.
“The cause of this voluntary recall is due to the identification of two separate confirmed counterfeit Xalatan 0.005% w/v Eye drops 2.5ml solution lots, with authentic Pfizer Lot numbers W6769 and AK4753, being distributed in the legitimate supply chain in the Nigeria market where the authentic Xalatan 0.005% w/v Eye drops 2.5ml solution with the same lot numbers been distributed.
Advertisement
“The use of the counterfeit Product Lots has a high probability to cause therapeutic failure and adverse events, such as infections. The potential risk to patients is considered high as patients will not be able to identify the counterfeit product.”
The product batches affected are those with lot numbers W67369; expiring 10/2020 and AK4753 expiring 10/2021
Pfizer said the recall plan is to immediately stop distribution and place the product lots into quarantine.
Advertisement
The company said the recall is being carried out with the knowledge and approval of the National Food and Drug Administration and Control Agency (NAFDAC).
TheCable has contacted Abubakar Jimoh, the agency’s spokesperson, for comments.
Add a comment