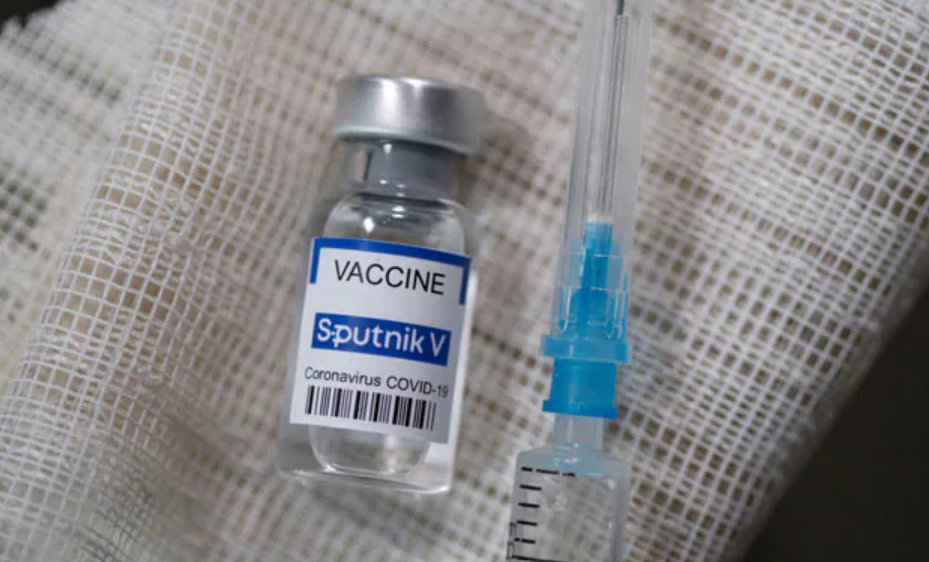
The National Agency for Food and Drug Administration and Control (NAFDAC) has approved Moderna and Sputnik V COVID-19 vaccines for emergency use in Nigeria.
Mojisola Adeyeye, NAFDAC director-general, disclosed this on Thursday at a news conference in Abuja.
Moderna vaccine is made by Rovi Pharma Madrid in Spain while Sputnik V is produced by Gamaleya National Centre of Epidemiology and Microbiology, Russia.
Until now, Johnson and Johnson as well as Oxford/AstraZeneca were already being administered in the country.
Advertisement
Adeyeye said the NAFDAC vaccine committee had been carefully assessing the vaccines and several others in spite of the approval by the World Health Organisation (WHO) for emergency use listing (EUL).
She added that all the COVID-19 vaccines that had gone through the process of approval had been certified for quality, safety and efficacy evaluation, a prerequisite for acceptance by COVAX facility.
She noted that NAFDAC spent 15 days to thoroughly examine the submission package of the vaccines to ensure that the benefits outweigh the risks.
Advertisement
“The EUL will allow Nigeria to receive supplies of the vaccines from the COVAX facility. COVAX is the pillar of the Access to COVID-19 Tools (ACT) accelerator’s jointly led by the Global Alliance for Vaccines and Immunisation (GAVI) and the Coalition for Epidemic Preparedness Innovations (CEPI) and the WHO,” Adeyeye said.
“Its aim is to accelerate the development and manufacture of COVID-19 vaccines, and to guarantee fair and equitable access to low-middle income countries (LMIC) of which Nigeria is one.
“NAFDAC also gives full review for vaccines that have not gone through EUL route. This mechanism is explained in our guidance developed by the COVID-19 Vaccine Committee.”
Adeyeye added that the pharmacovigilance unit of NAFDAC would conduct safety and monitoring studies on the vaccines to record the side and adverse effects following immunisation.
Advertisement
Add a comment