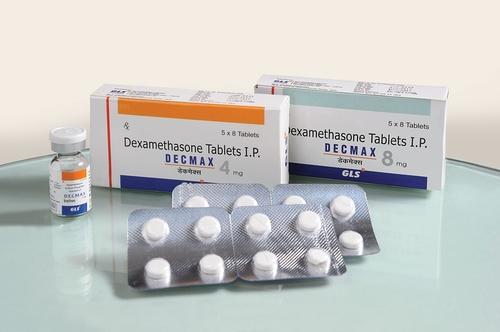
The World Health Organisation (WHO) has approved the use of dexamethasone for critically-ill COVID-19 patients.
Tedros Ghebreyesus, director-general of WHO, disclosed this during a press briefing on Monday.
On June 16, 2020, a team of medical researchers from Oxford University had said dexamethasone, commonly used for the treatment of rheumatoid arthritis and asthma, can speed up the recovery of high-risk COVID-19 patients.
Martin Landry, the lead researcher, had also said the drug costs £5 per patient and is available worldwide.
Advertisement
Speaking on the use of dexamethasone, WHO called for increased production and distribution, but emphasised that there is no evidence that the drug works for patients with mild symptoms.
“Although the data are still preliminary, the recent finding that the steroid dexamethasone has life-saving potential for critically ill COVID-19 patients gave us a much-needed reason to celebrate,” he said.
“The next challenge is to increase production and rapidly and equitably distribute dexamethasone worldwide, focusing on where it is needed most.
Advertisement
“Demand has already surged, following the UK trial results showing dexamethasone’s clear benefit. Fortunately, this is an inexpensive medicine and there are many dexamethasone manufacturers worldwide, who we are confident can accelerate production.
“Guided by solidarity, countries must work together to ensure supplies are prioritised for countries where there are large numbers of critically ill patients, and that supplies remain available to treat other diseases for which it is needed.
“Transparency and constant monitoring will be key to ensuring needs dictate supplies, rather than means. It is also important to check that suppliers can guarantee quality, as there is a high risk of substandard or falsified products entering the market.
“WHO emphasises that dexamethasone should only be used for patients with severe or critical disease, under close clinical supervision.
Advertisement
“There is no evidence this drug works for patients with mild disease or as a preventative measure, and it could cause harm.”
Before the approval by WHO, the Nigeria Centre for Disease Control (NCDC) had said use of the drug would be evaluated before it is allowed for COVID-19 treatment in the country.
“Please note that the Government of Nigeria has not validated or approved any treatment for COVID-19. In addition, the use of Dexamethasone for COVID-19 treatment has not been validated by WHO,” it read.
“We are aware of ongoing clinical trials conducted by scientists in the UK and will work with our sister agencies to evaluate this emerging data on the use of Dexamethasone. We will inform the general public on outcomes following scientific review and validation.”
Advertisement
Add a comment