The European Union (EU) says it will speed up COVID-19 vaccine production and distribution on the continent “over the next few weeks”.
Advertisement
Charles Michel, president of the European council, said this on Thursday. He said the EU will also improve the ability to distribute the vaccine.
“We are trying to take an inventory of the work that has been done. Over the next few weeks we hope to step up and speed up the production and distribution of vaccines to member states,” Michel said after he chaired a meeting of the 27 member states.
“It’s absolutely vital of course that we keep on working to improve vaccine production in Europe, and improve our ability to distribute those to member states.”
COVAX announces vaccine delivery delays from India
Advertisement
The delivery of COVID-19 vaccines produced by the Serum Institute of India (SII) to low-income economies participating in the COVAX facility will face delays in March and April, according to Gavi, the Vaccine Alliance.
COVAX said it has notified all affected countries of the potential delays.
Advertisement
A statement by the group cited the government of India’s battle with a new wave of COVID-19 infections as the reason for the delay.
On Wednesday, India’s ministry of health announced it discovered a new “double mutant” variant of COVID-19, as the country battles to contain a surge in cases.
Advertisement
“According to the agreement between Gavi and the Serum Institute of India (SII), which included funding to support an increase in manufacturing capacity, SII is contracted to provide COVAX with the SII-licensed and manufactured AstraZeneca (AZ)-Oxford vaccine (known as COVISHIELD) to 64 lower-income economies participating in the Gavi COVAX AMC (including India), alongside its commitments to the Government of India,” the statement said.
“Separately, the COVAX Facility has informed participants allocated AstraZeneca-manufactured doses of the AstraZeneca-Oxford vaccine that some of the first deliveries due in March are now set to take place in April.”
Advertisement
EU medicines regulator to examine blood clot links
European Medicines Agency (EMA) says it has asked a group of experts to provide their views on links to blood clots reportedly associated with some COVID-19 vaccines.
According to a statement issued on Thursday, the EU medicines regulator said the group will meet with the Pharmacovigilance Risk Assessment Committee (PRAC) on March 29, to discuss the possible reasons why some people developed blood clots after receiving a COVID-19 vaccine.
The group includes experts in haematology, cardiovascular medicine, infectious diseases, virology, neurology, immunology and epidemiology.
Pfizer begins vaccine trial for children between age of five and 11
Duke University researchers have started testing Pfizer’s COVID-19 vaccine for children under the age of 12.
At least two children have already got their first shot, according to CNN.
Pfizer is starting the trials first in children between five and 11 to determine the appropriate dose.
Vaccines will start with 10 micrograms. If the dose is tolerated, the trial will escalate to 20 micrograms and then 30 micrograms. An adult dosage of Pfizer vaccine has 30 micrograms.
Pfizer vaccine currently has an emergency authorisation from the US Food and Drug Administration (FDA) for 16-year olds and older.
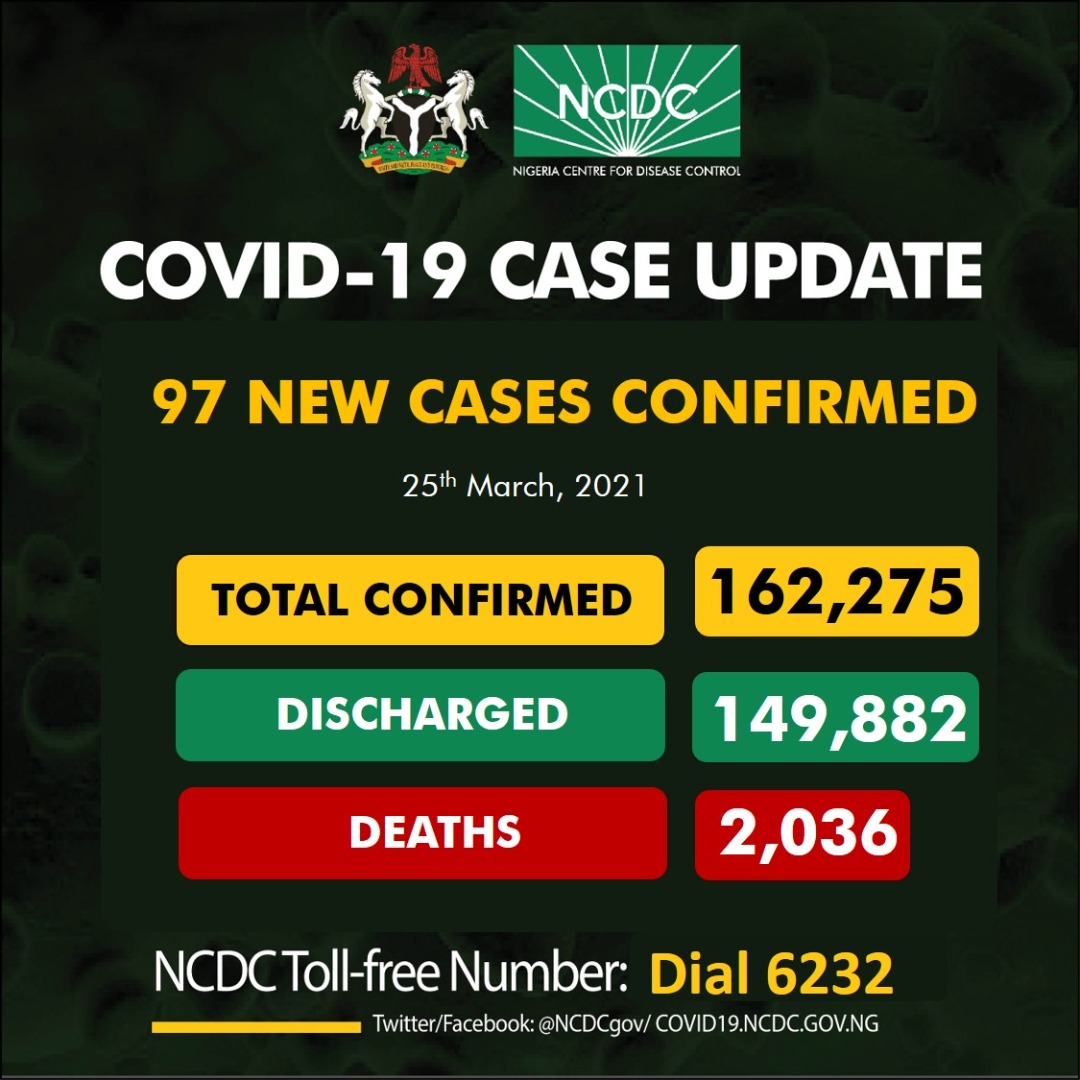
COVID-19 IN NIGERIA