Nigeria recorded 120 COVID-19 cases on Tuesday. Here are five updates about the pandemic this Wednesday.
Saudi embassy donates COVID-19 medical equipment to FG
The embassy of Saudi Arabia in Nigeria has donated COVID-19 medical equipment to the federal government to help curtail the spread of COVID-19.
According to NAN, the Kingdom donated relevant medical equipment worth $1 million to the government.
Advertisement
Twenty-three ventilators were said to have been donated while the date for the remaining equipment would be announced later.
The remaining equipment include “Surgical Sterile Gowns 25,000 pieces. Non-Surgical Sterile Gowns 125,000, Mask (KN95) 188,000, Surgical Masks 1, 606, 700 and Nitrile Gloves 9,500″.
“This is part of the donation by the Kingdom, which amounts to $500, 000, to support the international efforts aimed at combatting the COVID-19 pandemic,” a statement issued by the embassy said.
Advertisement
Indian capital running out of medical oxygen
The Indian authorities said hospitals in Delhi, the Indian capital, will start running out of medical oxygen by Wednesday.
Manish Sisodia, Delhi deputy chief minister, called for urgent help from the federal government saying “if we don’t get enough supplies by tomorrow (Wednesday) morning, it will be a disaster”.
Narendra Modi, Indian prime minister, said the government is working with local authorities nationwide to ensure adequate supplies of hospital beds, oxygen and anti-viral drugs.
Advertisement
“The situation was manageable until a few weeks ago. The second wave of infections has come like a storm,” he said.
“The central and state governments, as well as the private sector, are together trying to ensure oxygen supplies to those in need. We are trying to increase oxygen production and supply across the country.”
J&J to resume rollout of vaccine in Europe
Johnson & Johnson says it will resume shipments of its COVID-19 vaccine to the European Union, after the European Medicines Agency (EMA) recommended that the benefits of the single-dose shot outweigh its risks.
Advertisement
Last week, the use of the vaccine was temporarily halted by US health regulators after six cases of blood clots were reported. It also prompted the company to suspend its rollout in Europe.
J&J said on Tuesday that a new package label will include a warning on the risk of the rare side effect and instructions on how to recognise and treat it.
Advertisement
The company said it would restart shipments to the European Union, Norway and Iceland, and that it is working on restarting clinical trials.
“It’s an extremely rare event. We hope by making people aware as well as putting clear diagnostic and therapeutic guidance in place that we can restore the confidence in our vaccine,” Paul Stoffels, J&J chief scientific officer, said.
Advertisement
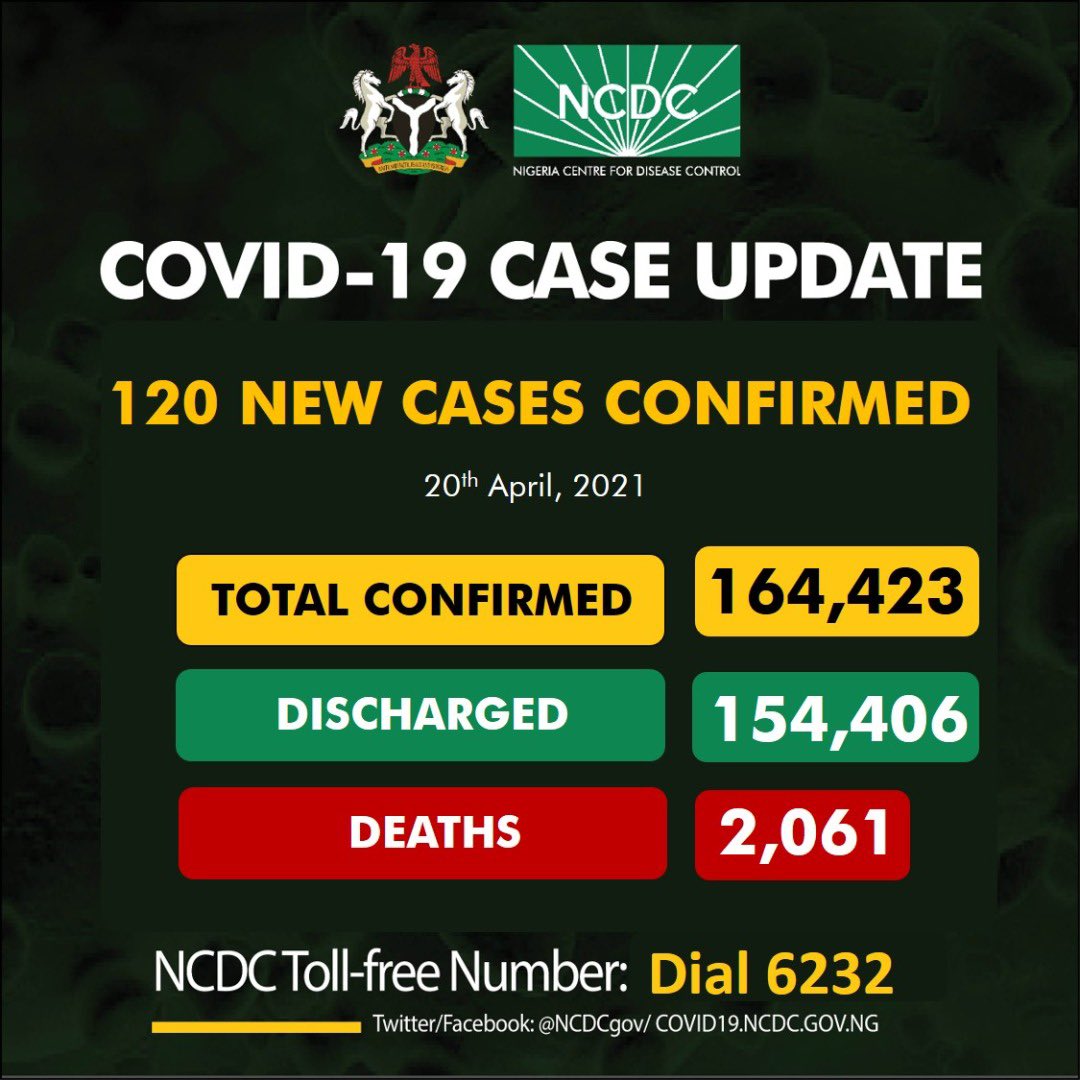
Nigeria records 120 infections
The Nigeria Centre for Disease Control (NCDC) recorded 120 infections on Tuesday.
Advertisement
The latest cases were reported in seven states and the federal capital territory (FCT), according to the agency’s update for April 20.
States that recorded new cases are Enugu (53), Lagos (22), Rivers (18), Ogun (8), FCT (7), Abia (6), Kano (5) and Bauchi (1).
The agency said infections recorded in Enugu on Tuesday were recorded between April 14 and 19.
The NCDC reported that 22 persons were discharged on Tuesday after recovering from the virus.
This brings the total number of discharged persons to 154,406.
The agency reported no COVID-19 death for the eighth consecutive day on Tuesday which keeps the total casualty figure at 2,061.
As of April 20, 1,870,915 samples have been tested while 7,956 are being managed across the states and the FCT.
Sweden to give different second vaccine dose to people
The Swedish health agency says people under the age of 65 who have received a first dose of the AstraZeneca vaccine will be given a different vaccine for their second dose.
Sweden paused the use of the AstraZeneca vaccine in March after reports of blood clots among people vaccinated with the AstraZeneca shot.
The country later resumed the use of the vaccine only for people aged 65 and above.
“People under the age of 65 who have already received a dose of Vaxzevria should instead be offered a second dose of so-called mRNA vaccine, such as PfizerBiontech or Moderna,” the health agency said in a statement issued on Tuesday.
COVID-19 IN NIGERIA