Johnson & Johnson on Tuesday said it has paused vaccinations in all clinical trials while it “update guidance for investigators and participants,” according to a statement from the company.
Advertisement
The announcement comes amid a recommendation from the US health agencies to pause the use of the Johnson & Johnson vaccine over blood clot concerns out of an “abundance of caution”.
“We have made the decision to proactively delay the rollout of our vaccine in Europe and pause vaccinations in all Janssen COVID-19 vaccine clinical trials while we update guidance for investigators and participants,” the statement said.
Advertisement
The company currently has several ongoing trials including an ongoing Phase 2a trial and a Phase 3b trial in South Africa.
Moderna COVID-19 vaccine more than 90 percent effective
Advertisement
The Moderna, drugmaker, says its COIVD-19 vaccine remains more than 90 percent effective for at least six months, according to a statement on Tuesday.
Moderna said preliminary results from its phase three trial shows that the vaccine has been more than 90 percent effective against all cases of the infection and more than 95 percent effective against severe disease.
Advertisement
“Vaccine efficacy starting two weeks following the second dose and based on the updated adjudicated cases remains consistent with prior updates, including greater than 90% against all cases of COVID-19, and greater than 95% against severe cases of COVID-19,” the company’s statement said.
WHO asks countries to halt sale of live wild animals in food markets
International agencies including WHO on Tuesday asked countries to suspend the sale of live wild animals in food markets, warning that they may be the source of more than 70 percent of emerging infectious diseases in humans.
The WHO-led team, which visited an animal market in Wuhan where the first human infections of COVID-19 were detected, had said the novel coronavirus was probably transmitted from bats to humans through another animal.
The interim guidance by WHO, the World Organisation for Animal Health (OIE) and UN Environment Programme (UNEP) asked countries to “suspend the trade in live caught wild animals of mammalian species for food or breeding purposes and close sections of food markets selling live caught wild animals of mammalian species as an emergency measure unless demonstrable effective regulations and adequate risk assessment are in place”.
“Animals, particularly wild animals, are the source of more than 70 percent of all emerging infectious diseases in humans, many of which are caused by novel viruses. Wild mammals, in particular, pose a risk for the emergence of new diseases,” the guidance added.
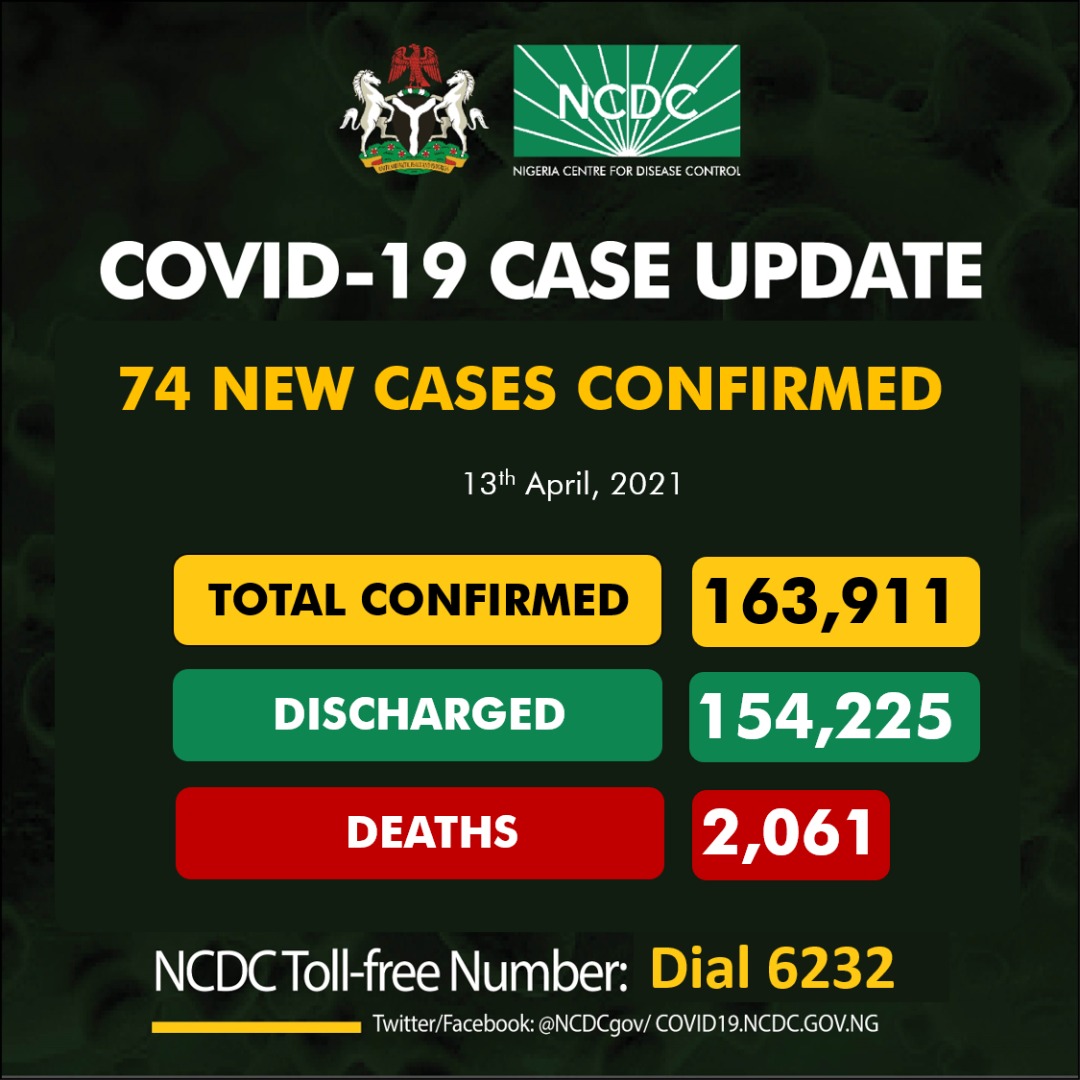
COVID-19 IN NIGERIA