Danladi's liquid substance
BY IJEOMA OPARA
As the Hepatitis B Virus (HBV) spreads across Nigeria, drug merchants are selling untested alternative medicines to patients, promising quick cures and putting them at risk of further harm.
Gertrude Inyang, an alternative medicine practitioner, sat behind the glass table in her office in Abuja on July 17, 2024.
She nodded her head at intervals as she listened to the reporter seated across the table, posing as a chronic HBV patient in search of a cure.
Advertisement
“We cure, we don’t treat,” Inyang said during the consultation. “We don’t manage such conditions; we treat them holistically.”
As Inyang spoke, she listed different terminal ailments which she claimed could be cured at her facility using alternative medicines, including all kinds of hepatitis, cancer and sickle cell disease.
“Is there anything that God cannot do?” she asked. “If you go to a pharmacy and pick any medication from the pharmacy, you must see side effects. Here, we are talking about nature. We flush the system. All the good organs, we activate. We have devices to activate, flush, and renew the organs.”
Advertisement
According to the World Health Organisation (WHO), alternative medicine, often referred to as complementary medicine, includes a wide range of health practices or substances that differ from orthodox medication and are not fully part of a country’s healthcare system.
Ayurveda, herbal medicines, and acupuncture are common forms of alternative medicine. Although most of them are derived from tradition, safety concerns regarding dosage and regulation exist. Sometimes, their efficacy is not backed by scientific evidence, leaving room for quacks to mislead patients.
For emphasis, Inyang shared “success stories” of patients who received treatment at her facility.
“This person went to the US every six months for 13 years. In the end, he had gone through radiotherapy, chemotherapy, and about three surgeries,” she said.
Advertisement
“The doctor told him: ‘Just go back, eat everything you can, and wait for two weeks.’ In fact, he was here yesterday. He is driving his car; he has gone back to his business; he is normal. They’ve been doing chemo to get it, but all of it didn’t work. But he came here, he got recovered. They gave him 2022, the first week of December, to die. But this is 2024.”
Inyang attributed the “successes” to the grace of God but pointed out that her treatments were costly, and eliminating HBV, a viral infection that attacks the liver, could take up to three months.

HBV is commonly transmitted through contact with blood, sharp objects, sexual intercourse or during childbirth.
When a patient gets infected, the virus can be acute or chronic. The immune system often clears acute infections within six months. Where the infection persists, it may progress to chronicity, but not all cases require treatment.
Advertisement
Jireh Makpu, a gastroenterologist at the Jos University Teaching Hospital, said treatment is required for patients with active HBV. Inactive cases only need to be monitored. Although the infection is treatablthere is currentlyently no known cure.
According to the WHO Global Hepatitis Report 2024, Nigeria had nearly 14.4 million HBV infections in 2022 and more than 46,000 deaths from the virus in the same year. Nigeria and nine other countries account for nearly two-thirds of the estimated global disease burden of viral hepatitis. Many drug merchants market untested products as cures for the ailment.
Advertisement
Inyang recommended five different therapy sessions in addition to oral medication. They cost N30,000 daily and involve daily exercise for at least 25 days.
This means, as of July 2024, HBV patients seeking a cure at Inyang’s facility would spend at least N750,000 for therapy within the first month of treatment, excluding the cost of oral drugs.
Advertisement
HBV tests are usually conducted by lab analysis of blood samples collected from patients. Still, Inyang told the reporter that a holistic test would be conducted with a Quantum Magnetic Resonance Analyzer (QMRA) machine to analyse all the organs, identify those that were failing, and determine which medicines to administer.
Some researchers have described the machine as lacking scientific backing, and experts argue that its results are unreliable.
Advertisement
“As far as I know, I am not aware that it has been validated for any of these things,” Makpu said of the QMRA. “As a scientist, I cannot recommend it for people who want to test for Hepatitis B. A blood test is still the way to go.”
The QMRA device on Inyang’s table looked like a medium-sized briefcase connected to a computer. She charged ₦25,000, then signalled a staff member to conduct the test.
He instructed this reporter to place her right palm on the beeping machine. After one minute, the results appeared on the connected laptop screen.

For about 15 minutes, Inyang studied the screen and took notes. She read out a long list of diagnoses, claiming that the reporter suffered from cardiovascular issues, liver problems, insomnia, an irregular menstrual cycle, and other ailments.
Inyang asked for a deposit of N250,000 to commence treatment. Citing harsh economic realities, this reporter pleaded to purchase only some of the prescribed products and return for the others later.
Inyang then recommended three products for a start: Cleanser, a transparent capsule filled with yellowish powder, Liver Care, a green capsule with a similar yellowish substance and Mira Tab, made up of spherical white pills and circular green pills.
UNVERIFIED NAFDAC NUMBERS

Although WHO recognises that traditional or alternative medicines are beneficial to healthcare, they may only be administered following rigorous clinical trials to prove their safety and efficacy.
In Nigeria, the Nigerian Agency for Food and Drug Administration and Control (NAFDAC) conducts clinical trials, after which registration numbers are issued to show that the drugs have been approved for use and are not harmful. However, the agency has repeatedly said that NAFDAC numbers do not certify that a drug actually does what it claims to do.
One of the three products prescribed by Inyang, Liver Care, had no NAFDAC registration number. The other products, Cleanser and Mira Tab had “A4-6889” and “A4-6695” printed on the labels as NAFDAC numbers, but there were no records of both on the agency’s Green Book portal, a database for registered drugs in Nigeria.
Despite these irregularities, Inyang told the reporter to stop taking orthodox medication prescribed by qualified medical doctors and focus only on her products. She prescribed eight tablets of Mira Tab and two and three capsules of Liver Care and Cleanser for the first dose.
“Start with three capsules,” she said, holding up the cleanser container. It will make you [have] loose stool… but if you do not go, the next day, take four.”
A BROWNISH LIQUID SUBSTANCE
About 22 kilometres from Inyang’s office, another practitioner, Alfred Danladi, operates Damla Herbs Resources, where he also offers cures for various ailments, including HBV.
“If you are going to follow synthetic, that is orthodox, you will be taking medication, and at the end, you end up having liver cirrhosis,” Danladi, the organisation’s managing director, said during a visit to his office.
He recommended the QMRA test after this reporter told him she’d been diagnosed with chronic HBV. When the test was concluded, Danladi said the reporter had developed resistance to orthodox medication. He assured that his treatment, estimated to cost N250,000, would eliminate the virus.
Again, this reporter opted to purchase only some of the products. Reaching behind a curtain that demarcated his office, Danladi produced two transparent bottles containing a murky, brown liquid.
The bottles had no labels showing names or NAFDAC numbers. Danladi warned that the bottles must never be kept on the floor during the treatment. But by the back door of his office, herbs, suspected to have been used in preparing the substance, lay all over the bare, dusty floor.
He also handed this reporter a transparent disposable cup with masking tape on its side to indicate the dosage.

Danladi said the brown liquid and other prescribed herbal substances, should be taken for four weeks. At the end of the period, patients are to avoid every type of medication for the next two months, then proceed for an HBV test, which, he assured, would return negative.
According to the US Centers for Disease Control and Prevention (CDC), leaving chronic HBV untreated can lead to liver cancer, cirrhosis or other damages. Sometimes, patients die.
While the infection causes the liver to be constantly exposed to injuries, many people remain asymptomatic until severe damage has been done.
Akeem Muraina, a 58-year-old Lagos resident, did not know that he had contracted HBV and remained asymptomatic until he fell severely ill in June 2024.
He started feeling unwell some days before the 2024 Eid-el-Kabir celebrations but put off going to the hospital until after the festivities.
“He told me that he was not feeling too well and did not want to go to the hospital, so he didn’t break down while we were celebrating in order not to bring our spirits down,” Muraina’s son, Imran Ridwan, said.
But Muraina’s health degenerated very quickly. He lost his appetite and vomited continuously. In less than a month, he lost considerable weight. After several hospital visits and a series of tests later, he was diagnosed with liver cancer.
His family struggled to raise the N26 million required for his treatment, and on Sunday, 13 October, Muraina died.
Desperation for a cure and the high cost of receiving medical treatment in Nigeria, where more than half the population lives in poverty, are some of the reasons patients turn to untested alternative medicine. Still, the use of such unregulated substances can be harmful.
Makpu said although most medicines are obtained from plants, the indiscriminate use of herbs for HBV treatment could have adverse effects on patients.
“Most times, people are given things they do not know, and many times, they do not even ask what the components of these things are,” Makpu said. “Those things most times damage the liver, and you’ll find them coming back worse…one can have what we call a hepatic coma. You find that it can be triggered or precipitated by herbal medications.”
To determine the content and safety of the products, Inyang’s Cleanser and Danladi’s liquid substances were sent to the University of Lagos Consultancy Limited (UNILAG Consult) laboratory, Lagos, for analysis.
HIGH PAH LEVELS, HEAVY METAL PRESENCE INCREASE RISK OF CANCER, ORGAN DAMAGE
Both samples had high levels of Poly Aromatic Hydrocarbons (PAH). These are organic compounds in the environment, water or food formed through human activity like burning coal, gas or industrial emissions.
Human exposure to PAHs can occur through smoking, eating or inhaling contaminated air. Prolonged exposure has been linked to respiratory diseases, damaged reproductive systems and an increased risk of cancer.
The liquid sample purchased from Damla Herbs Resources contained 0.1 parts per million (ppm) of PAHs, two times higher than 0.05 ppm, the permissible limits for drugs, according to the results of the analysis.
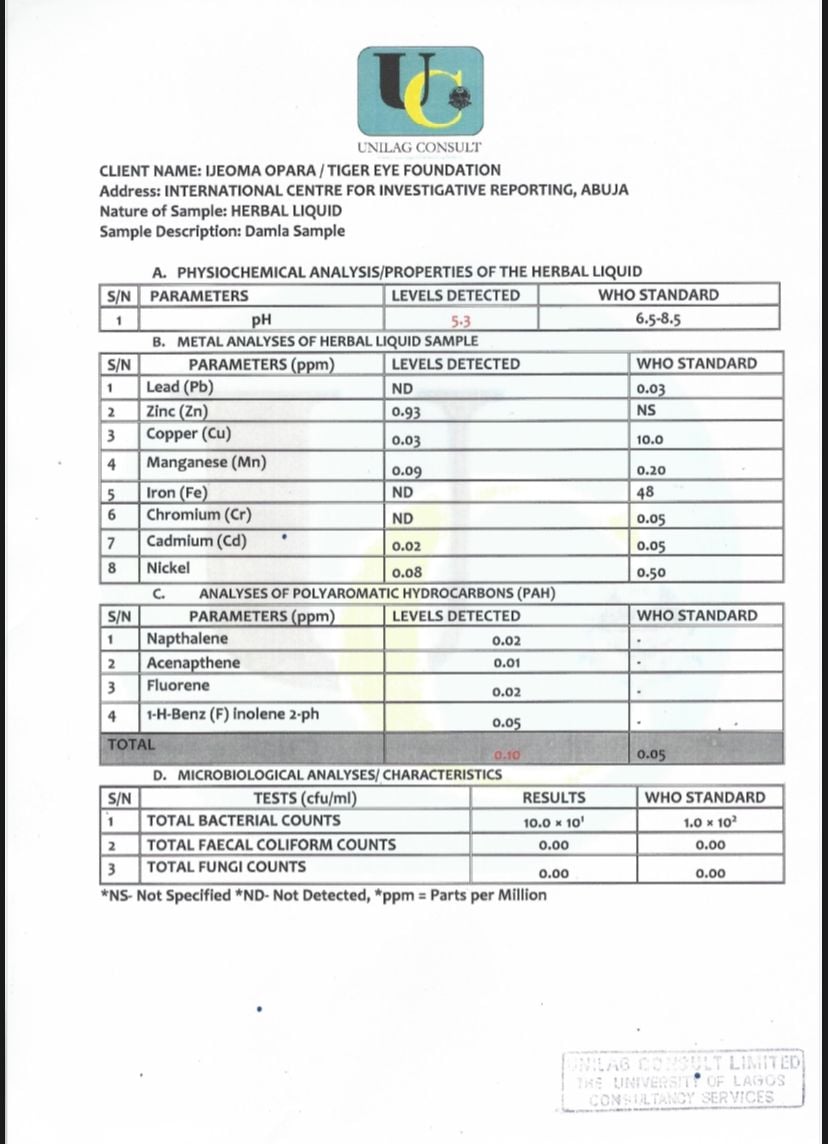
“Sometimes, by our interactions with the environment, these things get into our bodies in little quantities, and the body usually tries to detoxify or handle them,” Makpu said of PAHs. “But if they are coming in excessive quantities, they can overwhelm the body’s ability to handle them.”
An analysis of the Cleanser capsule at UNILAG Consult revealed PAH levels as high as 0.58 ppm, more than 11 times the permissible limits. According to the results, the levels of PAHs present in both samples may have other acute health effects, including liver and kidney damage and lung malfunction.
Heavy metals were detected in the Cleanser capsule, with Lead, Cadmium, Nickel and Manganese exceeding WHO standards.
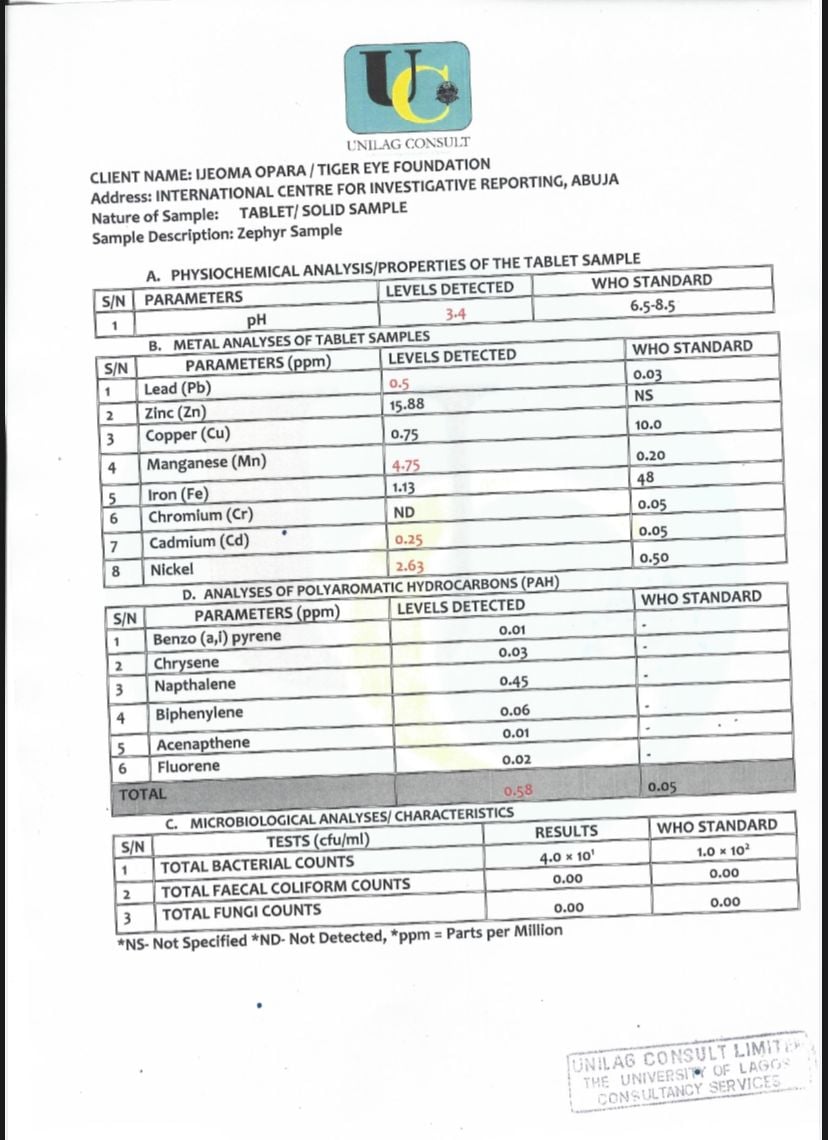
Lead exposure can cause gastroenteritis, an inflammation of the intestines and stomach, and damage to the brain and spinal cord.
“These heavy metals in blood samples can result in blood poisoning,” a laboratory technician at UNILAG Consult, Segun Gadimoh, said.
The lab results also showed that exposure to cadmium can lead to skeletal problems, while nikel exceeding recommended limits may cause skin allergies.
Reacting to the results, Makpu said such metals can significantly damage the liver, impairing its blood detoxifying function, even in patients without HBV.
“Manganese is one of those things thought to cause brain disease in patients with liver disease, and that’s because manganese can be deposited in parts of the brain and can worsen brain function in these patients,” he said.
This reporter reached out to Inyang with the lab results and questions on the sale of her products.
“I’m not a herbal practitioner,” Inyang replied. “I do not produce herbs. I am an acupuncturist… I don’t understand what you are saying.”
Inyang also denied selling drugs at her facility before ending the call.
A copy of the lab results and further questions were sent to her WhatsApp line. Although the messages were delivered and read, Inyang did not respond until press time.
Also, reacting to the results, Danladi denied giving any patient medication for hepatitis B recently and faulted the lab analysis.
“Where do you see ‘cure’ written on it?” he asked. “This medicine wouldn’t cure hepatitis B, only alleviate symptoms. Our company treats hepatitis B by having the patient take medication for 3 to 4 months under our supervision. Subsequently, he/she will check the viral load to see if there is improvement.”
Although Danladi sold two bottles of the liquid substance and directed that they be taken for a month, he said after the tests that the products expire after one week.
“This product only lasts for one week and may spoil if taken from Abuja to Lagos for lab testing,” he said.
He added that although the company did not have NAFDAC approval, it was registered by the Traditional Medicine Board under the Lagos State Ministry of Health.
A message sent to the board to confirm the registration had not yet been replied to at the time of filing this report.
MORE UNTESTED CURES
Despite possible harms associated with unregulated consumption of alternative medicine, the advertisement of unregistered products as cures to terminal or other ailments is quite common in Nigeria.
A social media user on Facebook with the username Spade has shared hundreds of videos claiming to have herbal cures for stroke, memory loss, fibroid, HBV and others.
In one of his videos, which had over eight thousand views, he claimed combining turmeric powder, bitter kola, and cloves could eliminate HBV. In a different video, he advertised a greenish liquid substance in a transparent plastic bottle with no labels, claiming he formulated the product from pawpaw leaves to cure HBV.
On X, an account with the username Ayodeji Awolumate posted an image of plastic bottles containing greenish liquid content. The caption read: “Permanent cure for Hepatitis B.”
Section 2(d) of the Herbal Medicines and Related Products Advertisement Regulations, (2021), prohibits the advertisement of “herbal medicines and any related products as a cure, prevention, treatment for any disease conditions listed in the schedule to these regulations or as may be prescribed by the Agency.”
Infective hepatitis and 64 other diseases were listed in the Act’s schedule.
In Gudu, Abuja, Love Egbo, a different alternative medicine practitioner, runs Fohow International Nigeria Ltd.
She said the organisation has its headquarters in Lagos but operates in several states, where agents like herself are recruited to market the company’s products. Egbo added that she has been in the business for over 13 years. She claims to be a medical doctor who stopped working in the hospital when she found more impressive results from Fohow products than orthodox medicines.
She also claimed that many people had been cured of HBV and similar ailments at her facility, including HIV. For Egbo, getting a cure often depends on the patient’s financial strength.
“It’s the money. Someone that I just gave a prescription because she has breast cancer… the prescription I made for her is six points something million [naira], but it is not what you will [pay] at once,” she said.
She said treatment could take three to six months, depending on the damage to the organs. Egbo recommended and attempted to perform a QMRA test, but the old laptop on her desk failed to power on when it was time for the test.
She urged the reporter to return with her HBV test results and prescribed drugs worth N288,000 without any test. The reporter paid N11,000 for only one of the products, Detox Plus, and promised to return for the others when financially capable.
Detox Plus’ content smelled like Inyang’s Cleanser capsules, though they had different containers. Both had similar health benefits on their labels. Still, while Inyang’s product came with a NAFDAC number that could not be verified, Detox Plus had a disclaimer: “NAFDAC has not evaluated these claims.”

She encouraged this reporter to continue with her orthodox medication and slowly transition fully into alternative medicine.
As the reporter made to leave, Egbo held her hands and said a prayer, fervently begging God to make the treatment effective.
In a subsequent interview, Egbo said the company received certification from NAFDAC to sell the product.
“When they were in the process of getting NAFDAC numbers, we had some products,” she said “So when they finally got all of them with numbers, we had some we shifted aside, but we still have the letters of the NAFDAC certification…the CEO gave us some letters to cover those ones, so when the product later had NAFDAC numbers, we were asked to return them so that she can restock our offices. But they are all the same thing,” she said.
She added that the products she sells only work as supplements and recanted her claims of having cures for HIV and similar ailments.
“What we give is to support the body system … we support such degenerative diseases,” she said.
She agreed to share a copy of the NAFDAC certification but did not do so.
HARMFUL PRODUCTS, MISLEADING CLAIMS FRUSTRATE PROGRESS OF ALTERNATIVE MEDICINES
Traditional medical knowledge has contributed immensely to healthcare globally.
More than 40 per cent of pharmaceutical products were formulated based on traditional knowledge, including landmark drugs like aspirin, the smallpox vaccine and the contraceptive pill, according to information on the WHO’s website.
In Nigeria, plant-based medicines are part of the nation’s cultural heritage, which many believe to have great prospects.
Martins Emeje, the director-general of the Nigeria Natural Medicine Development Agency, has called for the development of indigenous medicine to address dependence on increasingly expensive imported drugs.
Mojisola Adeyeye, NAFDAC’s director-general, has expressed her belief in herbal remedies on several occasions and urged practitioners to subject their products to clinical trials. She emphasised the need for more research into medicinal plants in Nigeria following the COVID-19 pandemic.
The agency has also repeatedly warned against the marketing or consumption of unregistered, substandard or falsified medicines in Nigeria, stating that they can have fatal consequences.
When contacted on December 9, Christiana Obiazikwor, NAFDAC’s public relations officer, who said she was on leave, referred the reporter to an episode of NAFDAC’s health awareness broadcast NAFDAC and Your Health where Adeyeye further spoke on steps to get approval and the need for more research into herbs.
“The DG is doing well on herbal… people should just come and register their products,” Obiazikwor said.
This story was produced with support from the Tiger Eye Foundation under the On Nigeria programme, funded by the MacArthur Foundation.