Rafiu Isa sells Sacra Herbs in Lugbe, Abuja
In Abuja, Nigeria’s federal capital territory, many residents consume ‘Baba Aisha Herbal Medicine’, a low-end herbal product that sells for just N100 and is touted to cure common diseases. In this five-month-long investigation, KEMI BUSARI scientifically probes the content of the concoction while questioning regulatory frameworks that allow its supply to thrive.
As the sun sets on the bustling streets of Abuja, a symphony of voices echoes through the residential communities; the voice of hope and healing emanating from horn speakers perched atop vehicles as herbal medicine vendors sing their healing song to all who will listen.
Among the various elixirs and tonics on offer, one potion reigns supreme: Sacra Herbs, known popularly as Baba Aisha herbal medicine. From thronging markets to busy intersections in Kubwa, Lugbe, Nyanya, Jabi, Kuje, Kurudu – in fact, every residential community in the federal capital territory – vendors of this medicine hawk their wares, promising to cure a litany of ailments for a mere N100.
Today, Baba Aisha, the sturdy figure behind the Sacra Herbs empire, takes centre stage in a spellbinding nine-minute advertisement blaring from the horn speaker balanced on a rickety Honda Accord car, permanently parked at Kurudu junction.
Advertisement
The ad opens with a devout prayer as the producer, who doubles as the voice-over artist, introduces himself as Dr Salisu Sani Na Wagini (Baba Aisha) and implores the heavens to guard his listeners against the twin curses of poverty and business loss.
With a flourish, shielding his apparent lack of fluency in the English language, Baba Aisha goes on to vaunt the many testimonies from satisfied customers while confidently listing the various maladies Sacra Herbs can cure: typhoid fever, malaria, stomach ulcers, rheumatism, waist pain, piles, and more. He urges his audience to take each of the 120ml bottles two times a day and to eat before consumption.
The ad then shifts to a litany of common symptoms, accompanied by boasts of the thorough research and expert craftsmanship that go into each bottle of Sacra Herbs. Then, he switches to a passionate appeal for people to turn away from the false promises of hospitals and modern medicine and instead trust in the power of Baba Aisha’s brew, especially for children.
Advertisement

“Even if you have the intention to go to the hospital, I, Dr Salisu Sani, say don’t go. Even if you go (they’ll) only say your children need drip or add them blood,” he said in the recorded ad. “Not everybody is capable to buy blood…but when you come here with small money, you can take our herbal medicine, and you’ll not get any sickness in your body anymore,” he added, leaving the audience with a sense of hope and conviction that his concoction is the answer to all their ailments.
Lurked behind these promises is an unsealed bottle of Sacra Herbs tainted with question marks. Questions about its origin, questions about its content, ingredients and mixing formula; questions about prescription and qualification of the producer; and, more importantly, questions about the regulatory framework upon which the product thrives.
To residents of Abuja and adjoining states, where the herbal product is prominent, Sacra Herbs come as a cheap and effective option whenever they are down with ailments; but months of rigorous investigation, which includes extensive laboratory testing of the product, put this in serious doubt.
THE UNREGISTERED CONCOCTION
Advertisement
What Baba Aisha referred to as medicine in the advertisement is a liquid mixture sold in a 120ml unsealed bottle. Each bottle contains a yellowish, minty liquid with a noticeable powdery residue. Our investigation reveals that the colour and amount of residue in each bottle are determined by retailers who add a black powdery substance after taking stocks of the pure yellow liquid.
The bottle also contains information on ingredients, a list of potential ailments cured, dosage, storage information, registration number, company details, telephone numbers and a picture of the producer. In the 14 locations visited across Abuja, the presentation and details on the items are the same.

One quick giveaway on each bottle is that the product carries not one, but two NAFDAC registration numbers.
The National Agency for Food and Drug Administration and Control (NAFDAC) is responsible for regulating and controlling the manufacture, importation, exportation, advertisement, distribution, sale and use of food, drugs, cosmetics, medical devices, chemicals and packaged water in Nigeria.
Advertisement
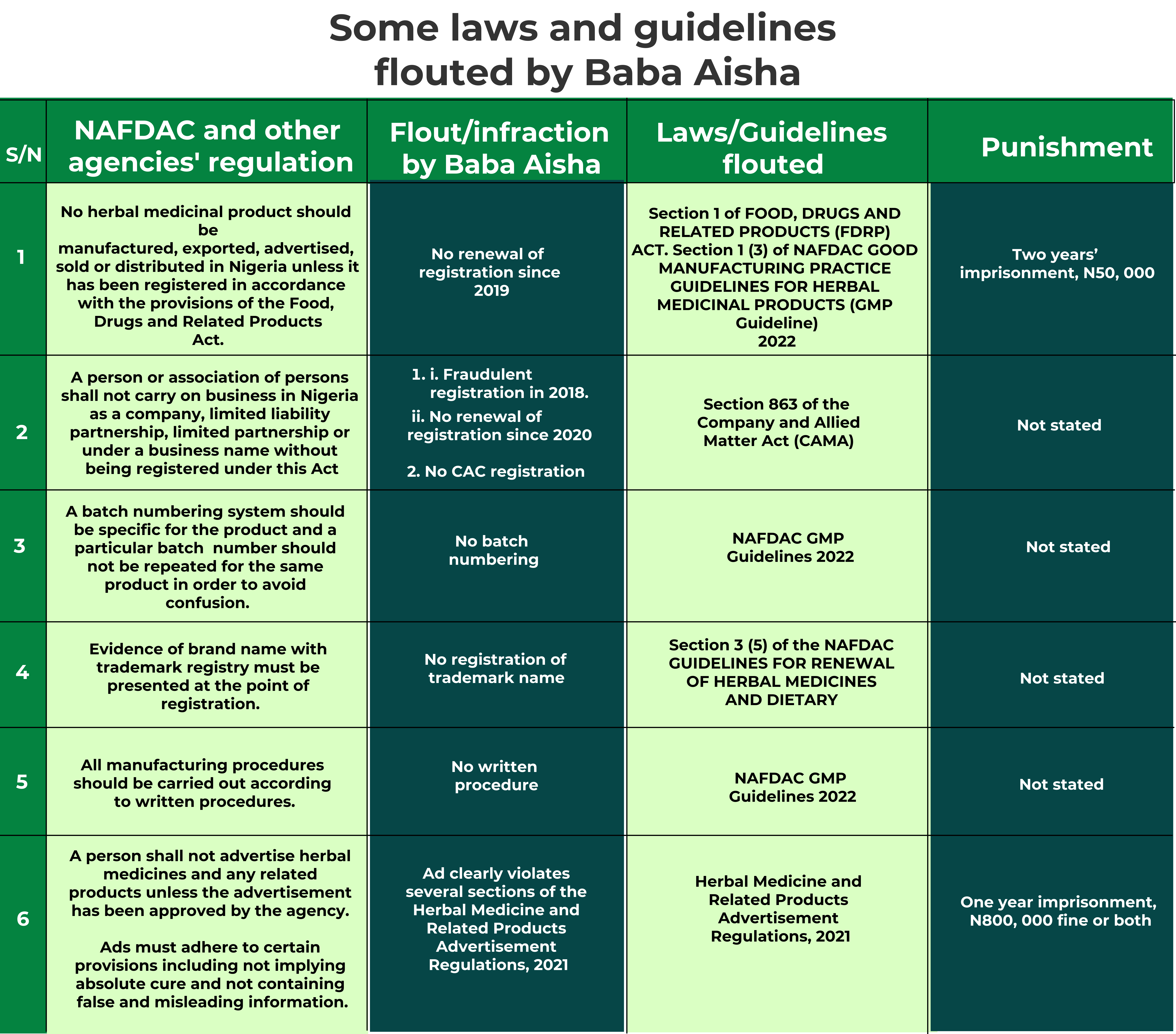
According to NAFDAC’s Food, Drugs and Related Products Act, no herbal medicinal product should be manufactured, exported, advertised, sold or distributed in Nigeria unless it has been registered. But here is Sacra Herbs with two registration numbers – A7-2551L and A7-2590L both tagged ‘NAFDAC REG NO’ on each bottle.
We checked the open portal of NAFDAC to confirm if both or either of the registration numbers is genuine; they were tagged as ‘fake’ on the agency’s search portal.
Advertisement

With some scepticism, we pressed further and then came across some reports that could confirm this revelation. In September 2017, NAFDAC impounded some Sacra Herbs products after the agency’s several efforts failed to get the producer to complete their registration and give full details of the products. Has Baba Aisha completed his registration since then? We wrote to NAFDAC to confirm.
In response, the agency said the herb was submitted for registration in January 2018 and the request was granted after “all the necessary procedures for registration were duly followed by the company and proper documentation provided.”
Advertisement
NAFDAC noted that it received a request for renewal of the registration in August 2020 but it refused to grant, as the application and vetting of the company’s facility for Good Manufacturing Practice (GMP) turned out “unsatisfactory”.

If regulations are followed, the concoction should already be off the market since the expiry of its registration but it remained a “saviour” for residents seeking cheap medication. Some of the users of the concoction described the stationary vehicles, where it is mostly sold in residential communities, as their “hospital”.
Advertisement
A few other users who cared to probe its authenticity said they were satisfied that it is registered with NAFDAC, probably ignoring or unaware of the fact that the concoction carries two registration numbers.
NAFDAC, in a written response, confirmed A7-2590L to be the correct registration number issued in 2018. The agency said the registration is yet to be renewed since its expiry in 2020.
“NAFDAC does not assign two registration numbers to one product”, the agency noted while disowning A7-2551L.

MORE REGULATORY INFRACTIONS, NAFDAC COMPLICIT
We spoke to over 20 users of the “medicine”, and most of them gave positive reviews. They will continue to use the “medicine” so far the regulatory agency has approved. However, NAFDAC may have breached this trust, our investigation shows.
Let’s start with a probe of the 2018 registration. According to NAFDAC’s guidelines for renewal of herbal medicines and dietary supplements, applicants seeking to register their products must fulfil some paperwork conditions; evidence of business incorporation by the Corporate Affairs Commission (CAC), evidence of registration of brand name with Trademark Registry of the Ministry of Industry, Trade and Investment, a comprehensive certificate of analysis by a quality control laboratory, among others. Our investigation shows that Sacra Multi-links barely fulfilled these criteria but was granted registration by NAFDAC.
Multiple searches on the CAC search portal for the company, Sacra Multi-Links Limited, returned a ‘search not found’ feedback, indicating that the company is not registered. After the open search, we contracted a lawyer to do due diligence on the company.
“The company is not registered with CAC,” said Olalekan Idowu, the lawyer, after a thorough check. “Under the law, you can’t even use your name for a business unless you’re registered.”
The law being referred to here is the Company and Allied Matters Act (CAMA), which in section 863 declares that “a person or association of persons shall not carry on business in Nigeria as a company, limited liability partnership, limited partnership or under a business name without being registered under this Act.”

We proceeded to find out if the company fulfilled the trademark criteria as required by law. Nigeria’s Trademark Registry, in response to our inquiry, noted the company filed for registration on November 23, 2016, but it failed to complete the process, even six years after. The registry noted that Sacra Multi-Links Limited “has not acquired the status of registration”.
Two lawyers who commented on the development said the company has at least two more stages – publishing of the trademark in the trademark journal and issuance of certificate — to follow to complete the registration. Both stages, they said, take a maximum of eight months to complete.
‘DON’T GO TO THE HOSPITAL’
In Nigeria, herbal medicine vendors are a dime a dozen, easily overlooked and forgotten. Perhaps the concoction wouldn’t have attracted this reporter’s attention but for the never-in-doubt advertisement that clearly set out to misinform the public.
In the nine-minute advertisement embedded below, Baba Aisha passionately urged the public not to visit or take their children to the hospital; he claimed hospitals only administer blood or drips for ailments; he gave prescriptions without prior academic or professional expertise and then claimed cure for several diseases – diseases with different origins.
Audio: Baba Aisha’s ad
To an average literate person, the advert comes as some kind of comedy, given the tone and the voice-over artist’s apparent struggle with the English language, but it is what several of his customers needed to hear to shun hospitals.
Alice John, 50, complained of pains in her eyes and other parts of her body in early 2022. Her first stop was a community clinic. Her medical records showed she was diagnosed with glaucoma, corneal ulcer and hypertension. She was placed on treatment but the ailments persisted, prompting an alternative. The alternative came in the persuasive words of Baba Aisha blaring from the stationary vehicle at the junction leading to John’s house.
“They (neighbours) said the medicine they are shouting (advertising) is working… It’s God (who heals) but I trust this medicine,” she said, adding that she hasn’t visited the hospital since she started taking the concoction in June 2022.

The advert, according to NAFDAC’s Herbal Medicine and Related Products Advertisement Regulations gazetted in 2021, ought to be approved by the agency before use. The regulation states that such ads shall not state or imply that any herbal medicine is “safe” or has “guaranteed efficacy or special status”. It noted further that such ads should not contain false or misleading information, vague, unsubstantiated statements or suggestions of superiority over other products or any claim of a universal cure. Baba Aisha’s ad failed in all of this and more!
With 63 per cent of the 200 million estimated population multi-dimensionally poor and the National Health Insurance Scheme covering only three per cent, herbal medicines easily become an option for many.
It is difficult to put a figure to the circulation and consumption of Sacra Herbs but a series of interviews with retailers and users suggest hundreds of thousands of the bottles are sold monthly.

We wrote NAFDAC to ask if it approved the ad but the commission failed to respond to our inquiry. Meanwhile, the regulation empowers NAFDAC to initiate legal proceedings and enforce payment of fines or imprisonment upon conviction.
We could not ascertain if Baba Aisha submitted fake documents or if officials of NAFDAC colluded to issue him registration without fulfilling the minimum criteria. Whatever the case, health experts say the agency has failed Nigerians in its role of keeping the public safe by not conducting due diligence.
“People are not going to double-check a product that has a NAFDAC seal on them,” says Lawal Bakare, a dentist and public health specialist. “Because Nigerians trust NAFDAC, the commission needs to know that it puts a lot of responsibility on them.”
We wrote NAFDAC to provide us copies of the documents submitted for registration but got no response. We presented this evidence to the commission and asked questions about the registration process but, again, got no response.
Baba Aisha declined our request to have an interview with him, despite answering repeated calls.
As revealed, NAFDAC’s lack of due diligence has for years put the lives of millions of users of Sacra Herbs, arguably the most popular concoction in Abuja, at risk. But how significant are these risks?
WHAT’S IN BABA AISHA’S BOTTLE?
Odigie Okai was experiencing relapsing malaria sometime in 2018, forcing him to either visit the hospital or spend on medicines every two months. “It got to a point some neighbours asked why I was always going to the hospital; that I was wasting money; that have I not heard of Baba Aisha?” he said.
Heeding the advice, a desperate Okai bought three bottles of the medicine on a prescription of one bottle per day.

“I took the first one the first day; towards the evening, I started feeling pains in my stomach. The next day, I took the second bottle, and it took a turn for the worse to the point I collapsed,” Okai recounted the terrible experience.
Waking up in the hospital, Okai was informed he had malaria and typhoid and that his situation was aggravated by the concoction.
His condition is similar to that of Isa Ismaila, a tricycle rider who was prescribed a daily dosage of three bottles of Sacra Herbs mixed with salt to cure typhoid sometime in 2022. After taking the first bottle, Ismaila soon became heavily sweaty, drowsy and extremely uncomfortable. He had to resort to over-the-counter medicine for his condition to normalise.
“Throughout the night, I could not sleep; I will never take the medicine again,” he said of his experience.

How did a medicine taken to ameliorate a condition end up worsening the ailment? We sought answers in Mols & Sims, an independent biological and chemical laboratory at the Afe Babalola University, Ado Ekiti (ABUAD).
Upon contacting him, Olaposi Omotuyi, a professor of pharmacology and therapeutics, and his team agreed to investigate the potency of the medicine in curing the diseases claimed, starting with malaria. The team also sought to establish if Sacra Herbs are safe for human consumption. Twenty bottles of Sacra Herbs, purchased in four different locations in Abuja – five each — were dispatched to the laboratory for investigation.
“To do this (investigate anti-malaria actions of the medicine), what we would do is an in-vivo experiment in which animal models of malaria would be established and the drugs will be used as an intervention versus a control, like chloroquine in this case, to check out the anti-malaria activity,” Omotuyi, who recently led a team of scientists to discover Virucidine Liquid, an approved cure for COVID-19, said at the beginning of the test.
Parameters adopted by the scientists include the increase in temperature, loss of weight, loss of appetite, death associated with the malaria parasite and an investigation of vital organs of the animals.

The scientist divided the animals into five groups of eight mice each to include: a basal group not ingested or infected with anything; a group infected with malaria parasite without treatment; a group infected with the malaria parasite and administered chloroquine treatment; a group infected with the malaria parasite and administered low dosage of Sacra Herbs (30mg per kg); a group infected with the malaria parasite and administered high dosage of Sacra Herbs (100mg per kg).
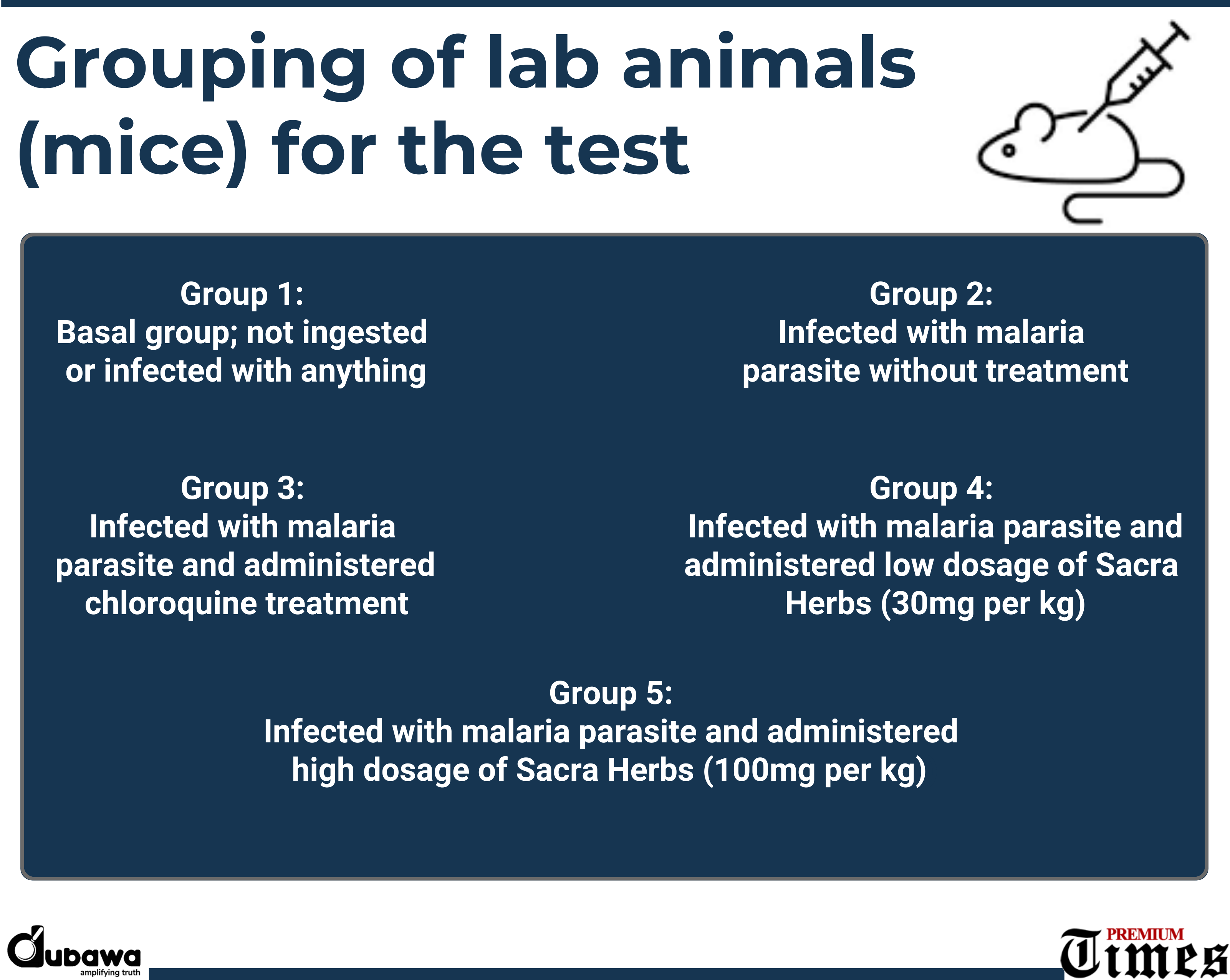
Summarily, after an experiment which took up to a month under set international standards, the team of scientists made the following key findings:
● Animals in the two groups administered low and high dosages of Sacra Herbs did not show any positive response to the medicine as a cure. Neither of the doses (100mg per kg nor 30mg per kg) has a curative effect on malaria infection. Therefore, the herbal medicine cannot cure malaria as claimed.
● Animals in the group administered chloroquine at 5mg per kg did not show adverse behavioural changes and were cured of the infection. Therefore, chloroquine is potent in the treatment of malaria.
● The plasmodium infection-associated loss of Packed Cell Volume (PCV) was not reversed under both doses of Sacra Herbs tested. That is when the blood levels of the animals began to go down as a result of the actions of the malaria parasite, the herbal medicine did not reverse the trend as expected of a cure.
● Animals administered Sacra Herbs at 100mg per kg suffered severe kidney and liver damage. Even those in the group infected with malaria and not administered a cure had better liver and kidney function. “This is a pointer to the possible damaging components present in the herbal mixture,” the laboratory report says.
● Animals in the 100mg per kg suffered low glutathione levels, weakening their immune system and making them susceptible to lung infections. Continuous intake for humans could lead to Acute Respiratory Distress Syndrome (ARDS), scientists say.
● All animals in the two groups administered Sacra herbs died within three to six days, meaning that the treatment was not successful. This also affirms that the medicine is a high safety risk to users.
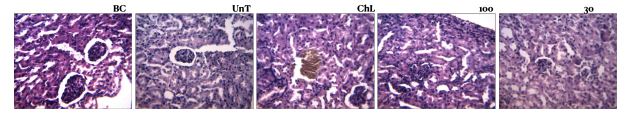
“It makes the kidney function almost impossible; it makes the liver function almost impossible; that means that anyone who takes this (Baba Aisha herbal medicine) is at a high risk of acute kidney injury, if not chronic,” Omotuyi said while analysing the result. “When it becomes chronic, one has to be thinking of a kidney transplant, and that does not come cheap. That is if you ever find a donor at all; ditto liver, ditto lungs. These are layman’s way of saying it is not safe to take this medicine as it is presently constituted.”
Although not outrightly established, users of Sacra Herbs such as Okai and Ismaila might have suffered from a breakdown of their vital organs, medical experts who analysed the situation say. Sometimes, this may lead to a total collapse of the kidney or liver, or even death, they add.
“We are seeing patients coming down with kidney failure, liver failure,” says Usman Bashir, a lecturer at the Department of Community Medicine, Bayero University, and a consultant at the Aminu Kano Teaching Hospital. “Our regulatory agencies need to really stand up, put control measures and even ban some of these herbal drugs because they are increasing our burden of disease, they are increasing the number of patients coming to hospitals and most of the time when they come, it is too little too late.”
According to the World Health Organisation’s (WHO) reports cited by many peer-reviewed journals, liver and lung diseases rank among the most-deadly ailments in Nigeria. This study and several others established that ingesting harmful herbs and roots accounts for a high percentage of hospital admissions resulting from kidney and liver diseases in the country.
To be specific on the figures, the Nigeria Association of Nephrology said about 20 million, accounting for 10 per cent of the population, live with kidney diseases. More disturbing, experts recently estimated that 45,000 Nigerians die from kidney diseases yearly.
The reality is grim for liver diseases. A February 2023 report by the WHO showed that more than 20 million Nigerians live with Hepatitis B, C, or both; yet more than 80 per cent of the people who have the disease do not know their status.
Some of these deaths are caused by cheap medicines, such as Sacra Herbs, taken to cure ailments. But there are more safety hazards lurking in a Baba Aisha bottle.
USERS AT RISK OF CANCER
Neither Zaradeen, the retailer of Sacra Herbs in Jabi; Isa, the retailer in Lugbe; Yusuf, the retailer in Nyanya, nor Ahmed, the seller in Kurudu, and other vendors could explain the yellowish nature of the concoction.
However, preliminary laboratory findings indicate that the toxicological burden of the concoction is in part associated with the dye included which was confirmed to be tartrazine yellow.
“According to the literature, tartrazine yellow dye is highly toxic to humans even at dosages considered safe,” the report notes.
Tartarzine is a colouring agent added to food and medicines to enhance their visual appeal. Several studies have shown that tartrazine’s metabolic conversion into aromatic amine (sulfanilic acid) could result in various disorders including anaemia, pathological lesions in the brain, liver, kidney and spleen, besides allergic reactions, tumours and cancer.
“On analysis of Sacra Herbs, we accurately determined that one litre of every Sacra Herb has about 5,000 milligrams of tartrazine yellow and in every 250ml bottle (approximately two bottles), it will come down to about 1, 250 milligrams and when calculated, everyone that has taken the herbs has taken about three times the recommended dose,” Omotuyi said, calling for an outright ban on the sales.
“It means everyone taking these herbs is exposing his (their) body to harmful substances of tartrazine yellow which might be responsible for all the organ destruction found in the animals used.”
He added that only 7.5 milligrams per kilogram is recommended daily for human consumption.
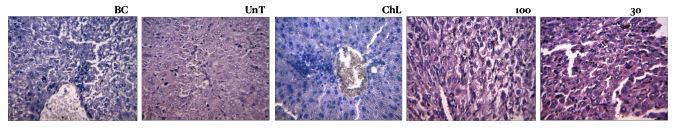
The scientists did not continue to check whether Sacra Herbs work for other ailments as ingesting the animals with the concoction creates an ethical issue since it is confirmed unsafe.
DYING AND SMILING
For Saleh Ahmed, a commercial tricycle rider; Mohammed Sani, a commercial motorcyclist; Alice John, a banana seller; and over 20 other users of Sacra Herbs interviewed for this report, the herbal medicine remains a trusted substitute for modern treatment.
“I prefer Baba Aisha (to visiting hospitals),” says Ahmed, who boasts of never taking any other medicine for more than two years now. “I will advise someone to take the concoction even if there is enough money to see a doctor.”
On surface analysis, the positive feedback from users is a direct negation of laboratory findings but experts say this could even be more dangerous.
“An average man is about 70kg. Most times, when you have an animal used (for lab test) and the animal dies within four days, it can (take) up to a year or two or longer time before they see any kind of damage (in humans),” says Oritoke Okeowo, a pharmacologist at ABUAD.
Other medical experts who analysed the situation gave the same feedback – the users are dying in instalments, unknowingly. They added that although the mixture might contain some potent ingredients, which explains why users get temporary relief, it is largely mixed haphazardly and constitute a health risk to users.

Okeowo fears if members of the public continue to consume Sacra Herbs and other harmful herbal products, there might be an increase in kidney and liver diseases and consequently, people’s inability to have transplants, which is currently estimated at N15 to N25 million for kidney and between N5 and N15 million for the liver.
When we got the initial results from the laboratory, we again visited John to relay the key findings to her and inform her of the dangers of the continuous use of Sacra Herbs. She told us she was still on the three bottles per day dosage in as much as she could afford it.
John, upon receiving details of the laboratory test, decided to run a kidney and liver test but later opted out for fear of the unknown.
“God has already washed it away. Any affliction devil wants to put in my body with that medicine, God will take it away for me,” she said, strongly dismissing her initial resolve to run a test.
Back in the laboratory, Okeowo is worried Baba Aisha’s high-dosage prescription is very harmful to users.
“We’ve given the mice 100ml per kilogram body weight, what this means is that for every 1kg, we are to give 100ml. If you multiply it, a human is about 70kg; that’s so much,” she said. “Even if something works, the number of times you have to take it might make you not follow up the dosage and you might not get the complete effect that you should get.”
Baba Aisha, in his ad, prescribed half a bottle of the herbal concoction for children irrespective of their age or body mass. Okeowo warned against such dosage.
“No matter the situation, I would say no child should be given this because they have smaller body weight,” she said. “If an adult is taking it and they are not yet collapsing, definitely it will be a lot worse for children.”
WHERE DOES BABA AISHA HERBAL MEDICINE COME FROM?
In October 2021, popular Twitter user, Daddy Odanz (@MrOdanz), in a banter-like manner, asked his over 100,000 followers who the “most popular herbal medicine doctor in Nigeria” was. More than a quarter of his respondents mentioned Baba Aisha.
In the comment section, they bantered on how Baba Aisha could already be brewing something for COVID-19, how his grammar-blundered ad lights up their evening journey from work and the experiences of people around them taking it. A few were curious to know why the herbal products are everywhere but no one knew the origin.
An address on the bottle of the concoction gave us a lead in our bid to unravel the location of Sacra Multi-links Limited.
Before embarking on the journey to Tafa, a town along the Kaduna-Abuja expressway, where the “medicine” is being produced, we contacted Mallam Hamza, who introduced himself as Baba Aisha’s brother.
After several calls, he agreed to meet this reporter for possible business collaboration and other discussions on the concoction. Hamza indeed showed up but he insisted he would not take this reporter to the location where Sacra Herbs is being produced. Several calls and pleas to Baba Aisha himself would not make either of them bulge.
Their insistence is easily understood; rather than an elaborate setting that guarantees standards and safety, the herbal concoction is produced in a residential building, which this reporter later found after locating the premises.

A herbal product hawker in Tafa, who doesn’t want to be mentioned to avoid being tracked, said there is no elaborate demarcation of the residence for production. The hawker added that the medicine is usually produced in bulk based on request by retailers.
Although we could not precisely ascertain as we weren’t granted entry, the building’s outlook fell far short of indications by NAFDAC’s GMP.
Herbal products are expected to be produced under certain standards prescribed by NAFDAC’s Good Manufacturing Practice (GMP). The GMP has many guidelines ranging from staff specialisation, premises marking, sanitation, storage, and equipment maintenance to water supply systems.
Baba Aisha would not comment on our findings despite answering multiple calls requesting his audience.
WHAT ABOUT REGULATION?
Herbal medicine practice in Nigeria is mostly unregulated and often requires no registration or scrutiny. For several years, individuals who parade themselves as “doctors” rip off the public with misleading advertisements without consequences.
At different times, the authorities have made attempts to regulate the sector, but the proceeds of these attempts have not yet materialised. One of such latest moves is that by Ibrahim Oloriegbe, a senator, who believes all herbal medicine practitioners should have a minimum qualification which should not exclude their ability to read and write.
In 2022, the lawmaker’s bill to establish the Federal College of Complementary and Alternative Medicine was assented to by the president and now awaits implementation by the Ministry of Health. Two years earlier, the federal government approved the bill for the establishment of the Council for Traditional, Alternative and Complementary Medicine Practice in the country.
“The college bill aims to improve the training of middle-level manpower for the practice of alternative and complementary medicine hence ensuring access to quality alternative medicines to Nigerians,” Oloriegbe says. “The council bill if passed, will ensure effective regulation of the practice of traditional and alternative medicine, eliminating quacks and ensuring the safety of the people accessing care.”
This objective would remain far-fetched without a common front by the herbal medicine practitioners. One association, the National Association of Nigerian Traditional Medicine Practitioner (NANTMP), is looking to formalise the workings of herbal medicine practitioners in Nigeria.
“The association is making an effort to curb the bad actors,” says Shaba Maikudi, the president of the association. “There is a committee in charge of this. They are in place to flush (out) and checkmate the bad eggs in the practice of traditional medicine because we are trying to meet up the WHO standard and without flushing quacks, we cannot be able to achieve this aim.”
“For herbal hawkers, we want to know the content of the herbs you are selling out to the public. We will now bring them in to educate them on how it can be safe for consumption. We also want to know if the herbs are not contaminated. We also want to make sure these people are registered so that we can know their area of specialisation.”
Maikudi confirmed that Baba Aisha is a member of the association, adding that a list of registered products will be made available on the Internet before the end of 2023. He hinted that the association, in conjunction with the Nigerian Natural Medicine Development Agency and the Ministry of Health, would soon start to test herbal products from a recently built laboratory sited in Lagos.
WHAT NEXT?
We sent copies of the laboratory report to NAFDAC, the Ministry of Health, the Consumer Protection Agency, the Standards Organisation of Nigeria and a few civil society actors, to inspire enforcement action.
As prescribed by NAFDAC’s regulations and other statutory laws, Baba Aisha and other vendors of harmful herbal products are liable to different punishments upon conviction.
NAFDAC failed to respond to our enquiry on what actions it would take and other questions regarding our findings.
Again, back to the laboratory, Omotuyi believes Baba Aisha’s idea can still be polished and properly implemented to guarantee safety.
“I am not so sure what has been put together to produce Sacra Herbs but the producer should also come open; let us know what components are there,” says Omotuyi. “He must work with scientists to help him establish safety first.”
But Baba Aisha has other ideas, he is confident of curing anyone of any race with his “medicine” so far you are mortal.
“If at all you’re a human being, you’re not a spirit, you’ll call us and tell us ‘this your medicine is working’,” he boasts at the peak of his ad, which attracted us to launch this investigation.
Add a comment