FILE PHOTO: A test tube labelled vaccine is seen in front of AstraZeneca logo in this illustration taken, September 9, 2020. REUTERS/Dado Ruvic/Illustration/File Photo/File Photo
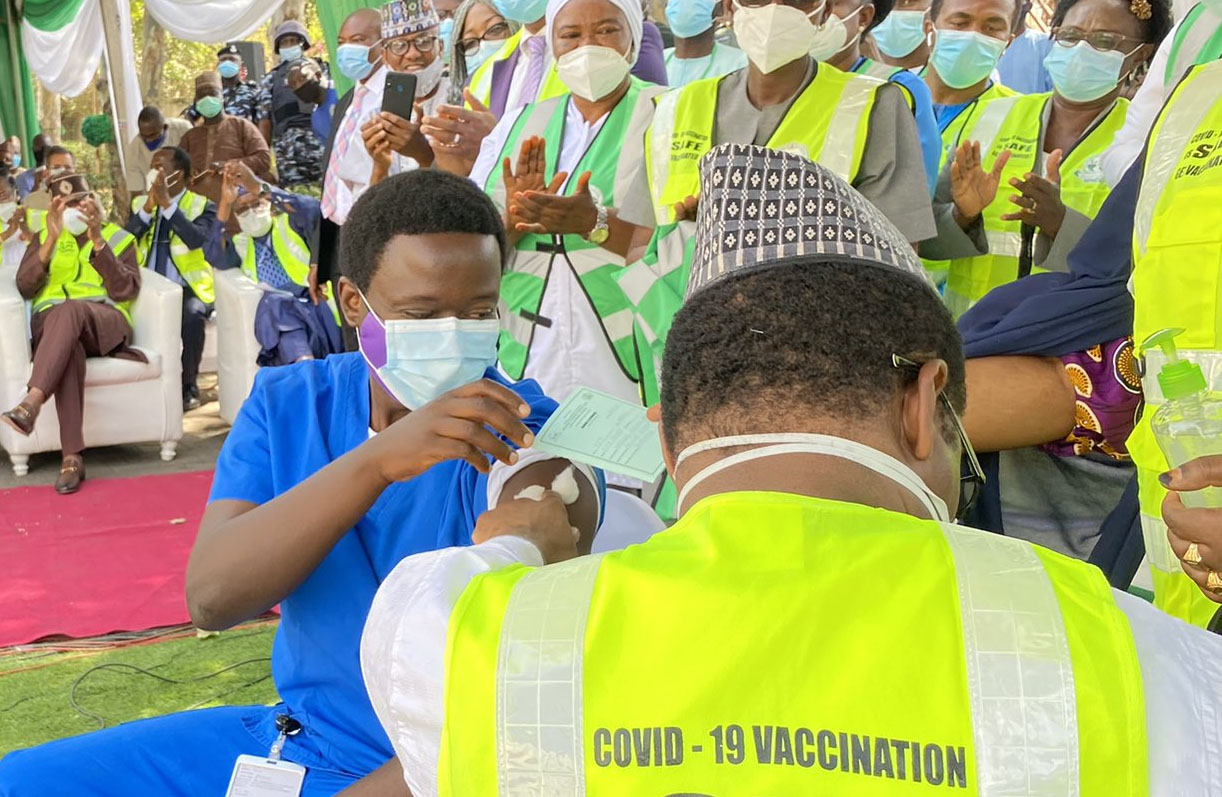
The National Agency for Food and Drug Administration and Control (NAFDAC) has certified the safety of the Oxford/AstraZeneca COVID-19 vaccine for use in Nigeria.
NAFDAC gave the final approval after last-minute tests were carried out ahead of the vaccine’s launch in Abuja.
Osagie Ehanire, minister of health, said at the ongoing launch that the agency gave the green light at some minutes past midnight on Friday.
Ehanire said the NAFDAC approval came after the test which lasted about 48 hours following the arrival of the vaccine in Nigeria on Tuesday.
Advertisement
He added that the launch would have been cancelled if the agency did not give the final go-ahead.
“The long-awaited day is here in which Nigerians can now join the global community to be vaccinated against the dreaded COVID-19 virus,” he said.
NAFDAC first approved the AstraZeneca vaccine for use in Nigeria on February 18.
Advertisement
The World Health Organisation had also said the vaccine “must-have” criteria for safety, and its efficacy benefits outweighed its risks.
Add a comment