Photo: Screenshot/BBC
The World Health Organisation (WHO) has granted prequalification to the MVA-BN vaccine, marking it as the first approved for mpox.
This development is expected to improve access to the vaccine in communities where the need is urgent.
The WHO prequalification service assesses the quality, safety, and efficacy of medicinal products.
If a product meets the necessary requirements and its manufacturing sites comply with WHO standards, it is prequalified and included on the list.
Advertisement
This status ensures global acceptance of the drug and builds confidence in its quality among healthcare providers.
In a statement issued on Friday, the WHO said the prequalification of the MVA-BN vaccine, produced by Bavarian Nordic A/S, was based on information submitted by the manufacturer and a review by the European Medicines Agency, the regulatory body responsible for the vaccine.
The MVA-BN vaccine is recommended for individuals over 18 years of age and is administered as a two-dose injection, with doses given four weeks apart.
Advertisement
The WHO said this prequalification would help facilitate timely and increased access to the vaccine, especially in areas experiencing mpox outbreaks, to reduce transmission and contain the virus.
“The WHO Strategic Advisory Group of Experts (SAGE) on Immunization reviewed all available evidence and recommended the use of MVA-BN vaccine in the context of an mpox outbreak for persons at high risk of exposure,” the WHO said.
Although the vaccine is not currently approved for those under 18, the WHO noted that it may be used “off-label” in infants, children, adolescents, and pregnant or immuno-compromised individuals.
“This means vaccine use is recommended in outbreak settings where the benefits of vaccination outweigh the potential risks,” the statement reads.
Advertisement
WHO also recommends administering a single dose of the vaccine in situations where supply is limited.
“Available data shows that a single-dose MVA-BN vaccine given before exposure has an estimated 76% effectiveness in protecting people against mpox, with the 2-dose schedule achieving an estimated 82% effectiveness. Vaccination after exposure is less effective than pre-exposure vaccination,” the statement added.
The WHO also highlighted the vaccine’s good safety profile, which has been demonstrated through clinical studies and real-world use during the ongoing global outbreak, which began in 2022.
The organisation stressed the importance continuous data collection on the vaccine’s safety and effectiveness, particularly in the context of new virus strains and the evolving epidemiology.
Advertisement
Tedros Ghebreyesus, WHO Director-General, hailed the prequalification of the MVA-BN vaccine as a significant milestone in combating mpox, particularly in Africa, where the virus has caused numerous outbreaks.
“This first prequalification of a vaccine against mpox is an important step in the fight against the disease, both in the context of the current outbreaks in Africa and in future,” Ghebreyesus said.
Advertisement
“We now need urgent scale-up in procurement, donations, and rollout to ensure equitable access to vaccines where they are needed most, alongside other public health tools, to prevent infections, stop transmission, and save lives.”
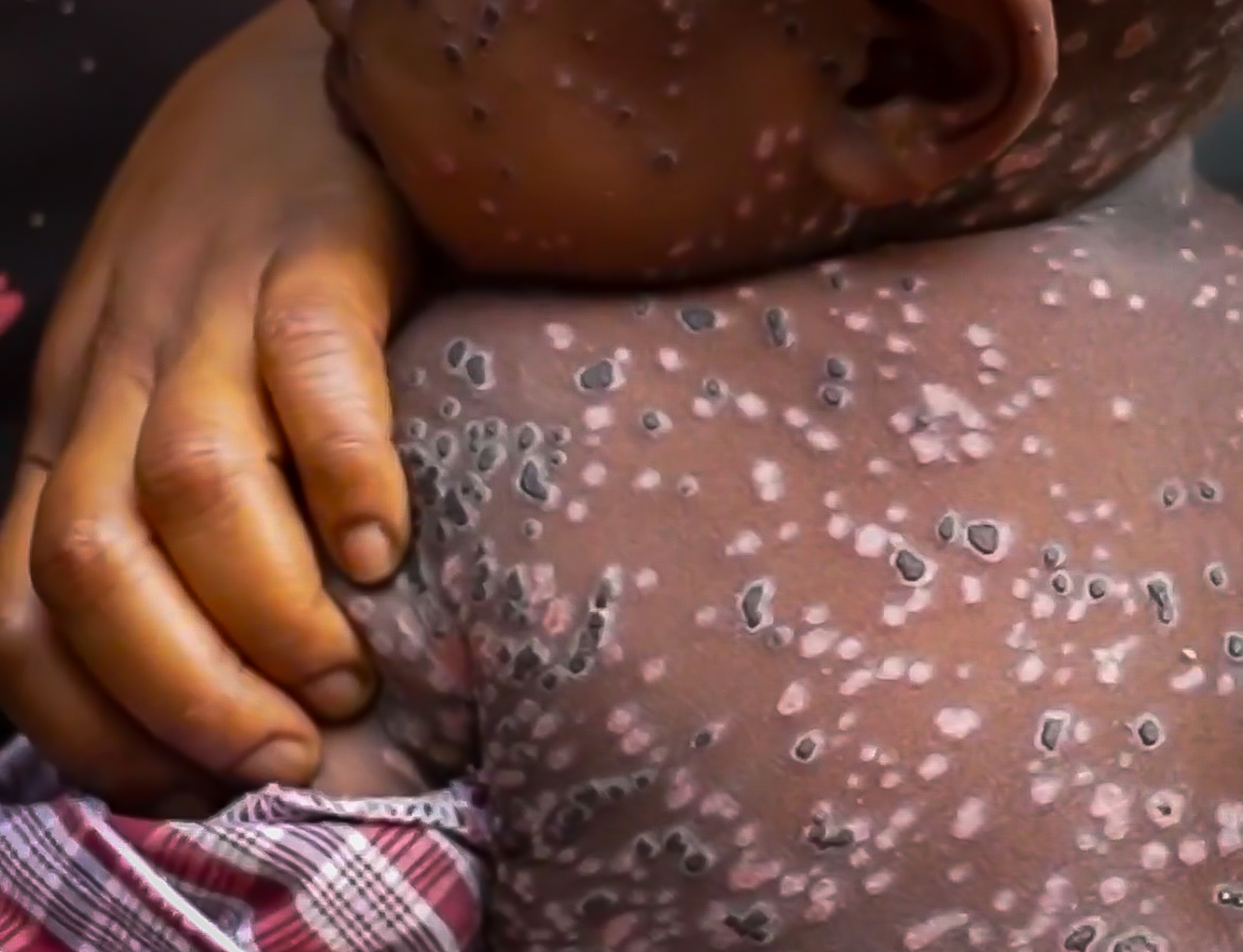
Mpox, which was declared a public health emergency of international concern by the WHO on August 14, has led to 25,237 suspected and confirmed cases, along with 723 deaths, in 14 countries across Africa in 2024 alone.
Advertisement