The World Health Organisation (WHO) has warned against the use of convalescent plasma in the treatment of COVID-19 patients.
In a statement on Monday, the organisation said the recommendation against was given by a WHO guideline development group of international experts.
But wait, what is convalescent plasma?
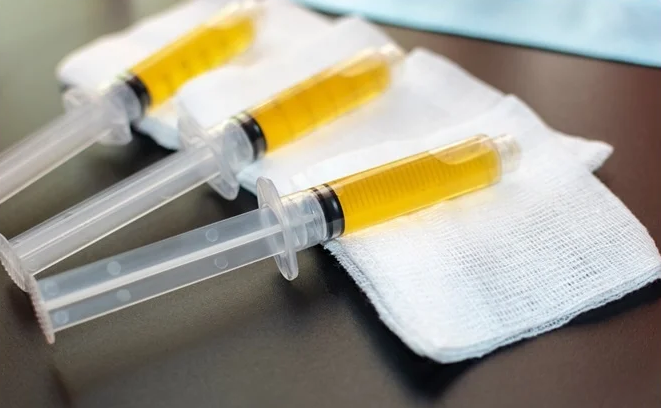
It is plasma taken from the blood of someone who has recovered from an illness — COVID-19 in this case — and transfused into a person still battling it.
Advertisement
Still confused about its purpose? Let’s break it down a little more. Plasma is a part of your blood. It’s light yellow and contains a ton of water — up to 92 percent.
When your body is exposed to foreign substances called ‘antigens’ (viruses, bacteria, fungi), its first response is to create antibodies, which are proteins that bind to this antigen to deactivate it. Feel free to call them your body’s soldiers.
Once the antigens have been defeated and you’ve recovered, the antibodies hang around for a while to fight the antigens, in case they come back. These antibodies are contained in your plasma.
Advertisement
Now, the idea behind the use of convalescent plasma for treatment is that plasma can be taken from your blood, and transfused into another person currently suffering from a disease caused by that antigen, so your antibodies can help that person fight the illness. Note that antibodies created for one particular antigen cannot work against a different antigen.
Interest in the use of convalescent plasma developed in the COVID-19 pandemic and numerous case studies have since been published, with varying results.
Why is WHO warning against it?
Since convalescent plasma seems like such a great idea, why is the WHO warning against it?
Advertisement
Well, the WHO said despite its initial promise, current evidence shows that convalescent plasma “does not” improve survival nor reduce the need for mechanical ventilation, and it is costly and time-consuming to administered.
It said its strong recommendations against it are based on evidence from 16 trials involving 16,236 patients with non-severe, severe, and critical COVID-19 infection.
“As such, the WHO makes a strong recommendation against the use of convalescent plasma in patients with non-severe illness, and a recommendation against its use in patients with severe and critical illness, except in the context of a randomised controlled trial (RCT),” the statement reads.
“To make their recommendations, the panel considered a combination of evidence assessing relative benefits and harms, values and preferences, and feasibility issues.
Advertisement
“The strong recommendation for patients with non-severe illness reflects the panel’s view that drug treatment in patients with a low risk of mortality and other important clinical outcomes is not justified.
“And although convalescent plasma should not be used routinely in any patients, regardless of how severely ill they are, the panel acknowledged that there was sufficient uncertainty in patients with severe and critical illness to warrant continuation of RCTs.
Advertisement
“They also noted several practical challenges, such as the need to identify and test potential donors, as well as collect, store and administer donor plasma, which they say further limits its feasibility and applicability.
“After thoroughly reviewing all the information, the panel judged that almost all well informed patients would choose not to receive convalescent plasma.”
Advertisement